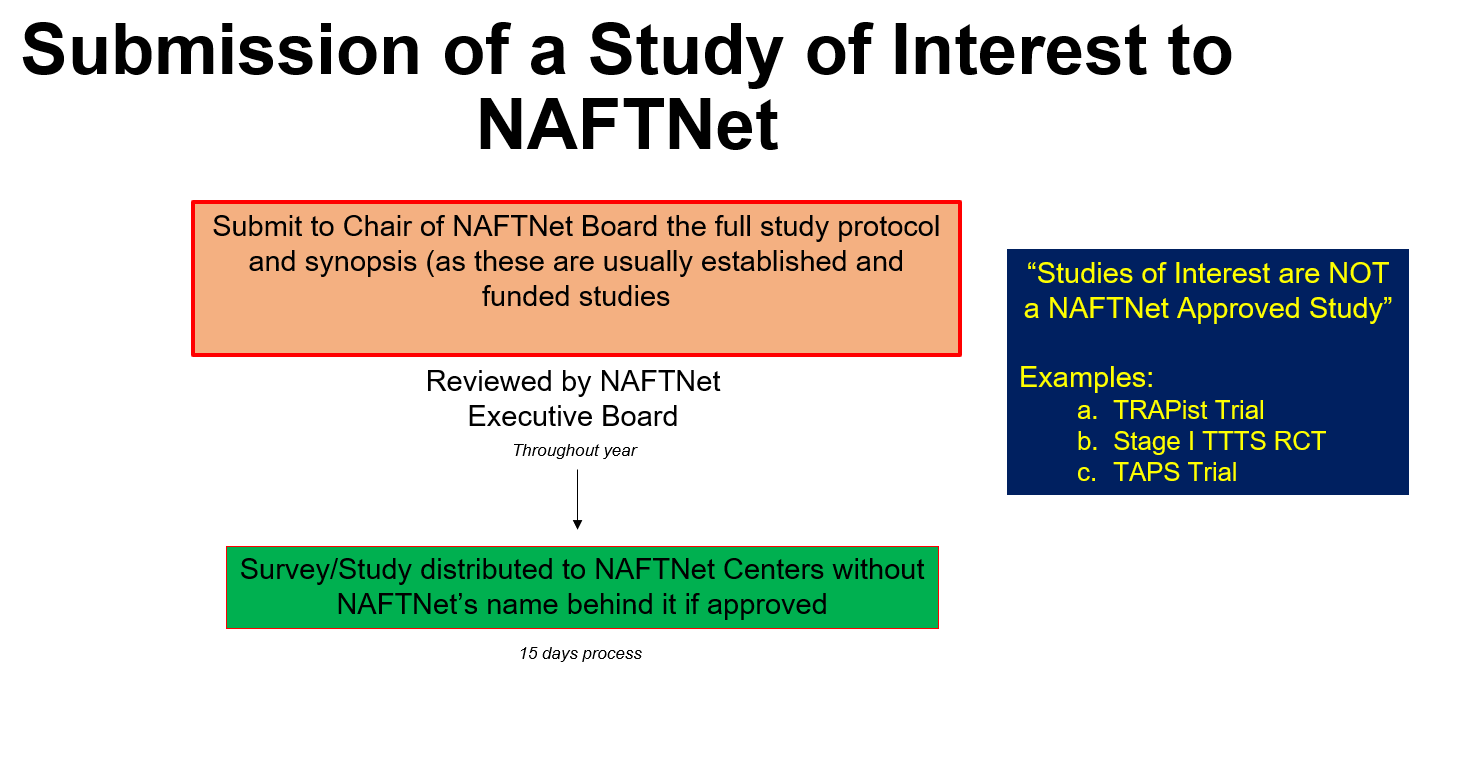
Submission of Research Protocols
Proposals for research studies can be submitted by any member of the Steering Committee. Guidelines for submission are outlined below. To review the SRS Committee Guidelines click here.
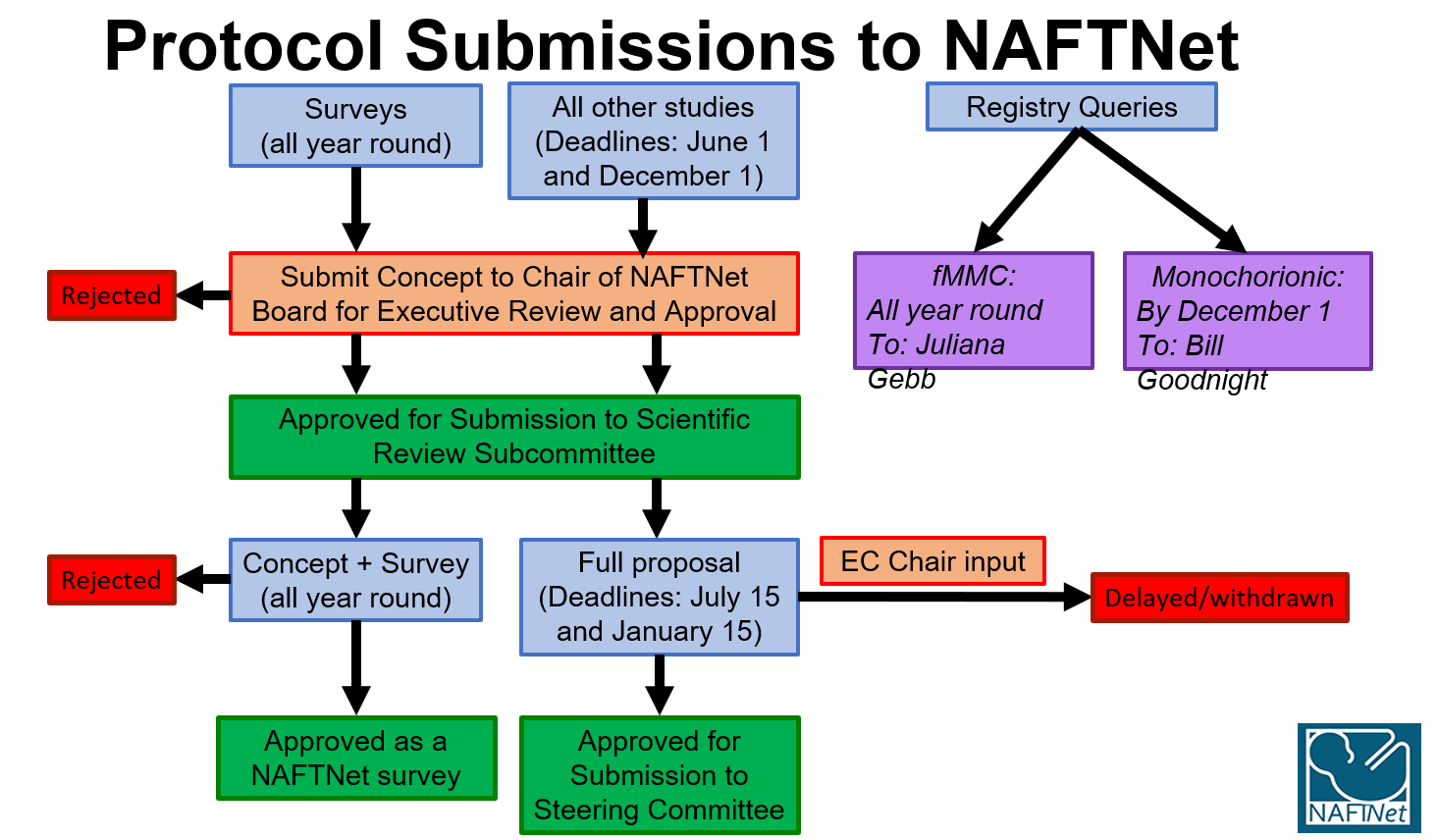 |
1. Surveys
Please email your survey proposal to info@naftnet.org.
- Proposals for surveys are accepted throughout the year.
- Submit electronically the concept proposal to the Board of Directors Chairperson (info@naftnet.org)
- Once approved by the Board of Directors, submit the approved concept proposal together with the proposed survey to the Scientific Review Subcommittee Chairperson. The Committee will aim to provide comments and a decision within 3 weeks of receiving the proposal. Approved surveys are active immediately and will be presented as FYI at the next Steering Committee meeting.
2. Registry Queries
Complicated Monochorionic Twin Pregnancies Registry:
See under Protocol 01/13 on the Research Protocols page for details on submitting a query for this registry.
Data requests are accepted once a year with a deadline of December 1.
Fetal Repair of Myelomeningocele Registry:
See under Protocol 01/12 on the Research Protocols page for details on submitting a query for this registry.
Data requests are accepted throughout the year.
3. Study Concept Proposal (All Other Retrospective & Prospective Studies)
For a template, click here.
- Submit electronically as an attachment to the Board of Directors Chairperson.
- Deadlines for Concept Form Proposal Submission are as follows:
- December 1 for the Spring Meeting
- June 1 for the Fall Meeting
- The Chairperson will distribute a blinded copy of the concept proposal to the Board of Directors for review. A simple majority approval moves the concept proposal forward to the Scientific Review Subcommittee.
- If the concept proposal is accepted, the applicant is notified within 15 days and invited to submit a complete research proposal to the Scientific Review Subcommittee for review and preparation for the Steering Committee presentation. Questions and concerns about the proposal may be included in the notification letter.
4. Research Proposal (All Other Retrospective & Prospective Studies)
For a template, click here.
- The PI of an accepted concept proposal will submit a research study proposal to the Scientific Review Subcommittee Chairperson, who will forward it to the Subcommittee members.
- The proposal must be received 3 months prior to next scheduled meeting:
- January 15th for the Spring meeting
- July 15th for the Fall meeting
- The Scientific Review Subcommittee will provide feedback to the Principal Investigator within 3 weeks of reception of the proposal and work with the PI to prepare the proposal for presentation to the Steering Committee. This review and preparation process will be completed at least 15 days prior to the meeting, to allow distribution and review by the Steering Committee members and preparation of an oral presentation by the PI.
- Oral proposal presentation will be in PowerPoint and not to exceed 20 minutes, followed by open discussion and questions with the Steering Committee. An anonymous vote will be conducted after the presentation. The Steering Committee may either 1) Approve as presented as a NAFTNet approved study (requiring 2/3 of votes for approval) OR 2) Support ongoing development with a working group and review at the next Steering Committee meeting (requiring a simple majority (>50%) of votes for support development or approval of the study) OR 3) Reject the proposal (if 50% or less of votes support development or approve the proposal).
5. After Approval of the Research Proposal
Approved Study:
If a research proposal has been approved as a NAFTNet protocol, the Board of Directors Chairperson will send a letter of Network’s commitment to participate to the PI to accompany any submitted grant applications or requests for funding. Information about participating NAFTNet Centers and NIH Biographical Profiles of the designated primary NAFTNet member from the centers will be available to the study PI to download from the NAFTNet website to accompany their funding application.
Supported Development:
For proposal obtaining support to move forward but requiring further revisions, the Scientific Review Subcommittee Chairperson will appoint a 4-person working group (chaired by one member of the Scientific Review Subcommittee) to support the PI.
There will be a conference call between the members of the working group within 30 days of the Steering Committee meeting to discuss desired improvements to the study. Ongoing feedback will be given to the PI by the working group following each revision until all issues are resolved and the working group feels it is ready for final presentation, aiming for completion of this process within 15 days of the next Steering Committee meeting.
The working group chair will provide the Scientific Review Subcommittee Chairperson with a progress report 2 and 4 months after the Steering Committee meeting, describing progress and challenges.
The PI will submit the final edited research proposal within 15 days of the next meeting for distribution and review by the full Steering Committee.
The PI will re-present the proposal to the next Steering Committee meeting outlining changes made to address the issues raised at the initial presentation. Following further discussion, the study proposal will be voted on for Network support. An anonymous vote with a 2/3 majority approving Network support is required. Otherwise, the study will not be a NAFTNet supported study.